XFEL: Team uses X-ray laser to watch electrons travel across molecule
Team uses X-ray laser to watch electrons travel across molecule
An international team including scientists from European XFEL has watched a process inside a molecule that could give insight into how to better harvest solar energy. For many years, scientists have been trying to find ways to convert energy from the sun into chemical energy that can be stored for long periods of time, similar to what happens in plant cells during photosynthesis. In order to do that, the researchers need to see how that conversion happens in model systems that are similar to promising compounds that could eventually be used to translate energy from solar to chemical on an industrial scale. This requires the ability to look deep inside the reacting molecule while the basic steps leading to the energy transfer take place. The now published results in the journal Nature Communications show unprecedented details about the movement of an electron across such a molecule.
Christian Bressler and his Femtosecond X-Ray Experiments (FXE) instrument group from European XFEL suggested a method of analyzing the X-rays given off by a previously energized molecule, or its X-ray emission spectrum, using an X-ray free-electron laser (X-ray FEL). X-ray FELs, such as the future European XFEL, are emerging new light sources capable of delivering ultraintense and ultrashort flashes of X-ray radiation. These facilities are extremely useful in this sort of study because they can reveal the activity of the electrons close to a molecule’s constituent atoms with the shortest exposure time and therefore highest time resolution possible today. Prior established methods, which are based on ultrafast optical laser technology, can only interact with the molecule’s electrons that are furthest from the atoms and thus located across several atoms simultaneously. With ultrashort X-ray FEL pulses, scientists can now follow the path of electrons from one specific part of a molecule to another specific part, combining available X-ray tools with the incredible time resolution of X-ray FELs, which allows the researchers to observe changes that are faster than one trillionth of a second. This means they can also see how quickly the electrons can transfer their energy—giving some indication of efficiency.
“The emerging possibilities offered by X-ray FELs permit us to gain new insight into how reactions proceed, so that we can better understand how quickly the excited electrons move along the molecule or how its internal structure changes”, says Villy Sundström, one of the co-authors on the paper and a professor of chemical physics at Lund University in Sweden.
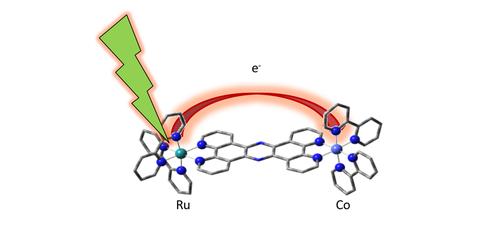
A molecular “bridge”, which chemists call a ligand, between both metallic centres serves as a waypoint for the electron. The scientists used an optical laser to excite the light-capturing ruthenium atom, and, using the short flashes of the SACLA X-ray FEL, they watched changes in the molecule as the ruthenium’s electrons moved across the bridge to the cobalt acceptor.
“You’re figuratively watching the electrons hopping down an energy staircase, from the ruthenium starting point, over the bridge, and finally to the cobalt atom”, says Bressler, who is a co-author on the paper.
The experiment also served as a proof-of-concept of studies of similar compounds that may be useful in fuel production in the future. The new information obtained from the present study lies in the details of the final steps, when the electron settles down inside the acceptor cobalt atom.
“We have studied this molecule in our university laser lab for years now”, says co-author Sophie Canton, formerly a member of Sundström’s lab in Lund and now a bridge scientist between Deutsches Elektronen-Synchrotron in Hamburg and the Göttingen Research Campus, specifically the Max Planck Institute for Biophysical Chemistry. “But we were unable to observe any direct trace of the electron transfer to the cobalt center due to its ‘optically dark’ nature. Even state-of-the-art ultrafast optical tools have some limitations that prevent us from getting the entire picture.”
Therefore, the new prospects of X-ray FELs should be attractive to future European XFEL users, who seek to understand better the elementary steps in molecular reactivity.
“Being able to look into the details of the efficiency of functional systems opens up wide perspectives”, says co-author Martin Meedom Nielsen, who is a professor at the Technical University of Denmark in Lyngby. “Future research targets range from catalysts for efficient photovoltaic devices to biologically relevant systems such as those used in photosynthesis.”
