XFEL: Finding the chink in corona’s armour
Finding the chink in corona’s armour
The COVID-19 pandemic resulted in millions of deaths. Despite an unparalleled collaborative research effort that led to effective vaccines and therapies being produced in record-breaking time, a complete understanding of the structure and lifecycle of the coronavirus known as SARS-CoV-2 is still lacking. Scientists used the biolabs and the SPB/SFX instrument at the European XFEL to study the main protease, or Mpro, of the virus to understand how it protects itself from oxidative damage. The results add key knowledge to our understanding of the workings of SARS-CoV-2 and the field of viral biology.
Between January 2020 and March 2023, over six million people died as a result of the respiratory disease COVID-19, and several hundred million were infected. The disease is caused by SARS-CoV-2, a coronavirus. “Coronaviruses are a group of RNA viruses that cause illnesses and diseases in mammals and birds”, explains European XFEL scientist Richard Bean. “However, despite their significant relevance for global human health, there is still a lot to learn about the structure and function of coronaviruses in general and SARS-CoV-2 in particular.”

Thomas Lane and colleagues in the control room of the SPB/SFX instrument at the European XFEL. (© European XFEL)
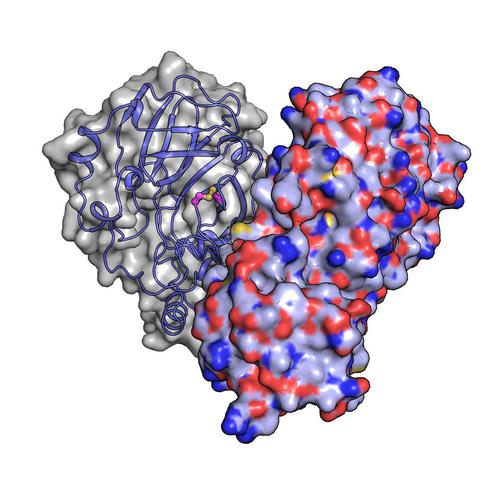
In a recent experiment at the SPB/SFX instrument at the European XFEL, Lane and colleagues used the intense X-ray beam to study Mpro. Several previous structural studies focusing on Mpro have highlighted a number of peculiarities. “Firstly, the protein forms a 3D structure known as a dimer when it is found in high concentrations”, explains European XFEL scientist Robin Schubert, who was involved in the experiment. “This structural habit seems to directly influence its activity—but we don’t know precisely why this is important for the virus.”
Alongside key insights into the 3D structure, recent studies have also hinted at the importance of cellular oxygen levels for protease activity.
“It seems that even mild exposure to oxygen decreases Mpro’s activity”, explains Patrick Reinke, also from CFEL. Indeed, in the presence of sufficient oxygen, turnover ceases altogether. But this process is reversible – if the oxygen is removed, the enzyme reactivates itself, suggesting the system has evolved protective mechanisms to survive in an oxidative environment. “Oxidative stress has been shown to regulate the function of other viruses, such as HIV”, Reinke adds. “It has been suggested that structural changes in the protease let it escape oxidative damage in oxygen-rich environments. However, we’re still unsure of how these protective mechanisms impact viral fitness.”
An understanding of the structure and lifecycle of the SARS-CoV-2 virus is essential to develop vaccines and therapies. (© CFEL)
“Our results show that the active site cysteine, which conducts the enzyme’s chemistry, can sneakily hide itself from oxidative damage”, says Schubert. Typically, oxidation can irreversibly damage cysteines. Upon oxidation, however, Mpro protects its most important cysteine by forming what is known as a “disulfide bond”, which buries it in the core of the protein structure. Then, if moved back into a safe, low-oxygen environment, the disulfide bond can break, revealing the active cysteine, which resumes its original function. “The experiments performed at the European XFEL reveal a picture of the protein in its hidden disulfide state, confirming it exists and uncovering how it works”, says Schubert.
“Mpro exhibits an unusually rich set of oxidation modifications, and our experiment adds a key piece to that story”, says Lane. The scientists are excited about what their data indicate and about their next steps. “Mpro is a linchpin of coronavirus biology and the premier target for anti-COVID-19 small-molecule therapeutics”, Lane adds. “The enzyme’s function has been shown to be regulated via both dimerization and oxidation, and it’s clear that these regulatory mechanisms are biophysically correlated. While our structures provide mechanistic insight into these properties of Mpro, we must now understand how regulation based on oxidative stress or protein concentration impact viral fitness. This will provide deeper insight into viral biology and hopefully open new opportunities to disrupt that biology with life-preserving medicines.”
Authors
- Y. A. Reinke*, R. Schubert*, D. Oberthür, M. Galchenkova, A. R. Mashhour, S. Günther, A. Chretien, A. Round, B. C. Seychell, B. Norton-Baker, C. Kim, C. Schmidt, F. H. M. Koua, A. Tolstikova, W. Ewert, G. E. P. Murillo, G. Mills, H. Kirkwood, H. Brognaro, H. Han, J. Koliyadu, J. Schulz, J. Bielecki, J. Lieske, J. Maracke, J. Knoska, K. Lorenzen, L. Brings, M. Sikorski, M. Kloos, M. Vakili, P. Vagovic, P. Middendorf, R. de Wijn, R. Bean, R. Letrun, S. Han, S. Falke, T. Geng, T. Sato, V. Srinivasan, Y. Kim, O. N. Yefanov, L. Gelisio, T. Beck, A. S. Doré, A. P. Mancuso, C. Betzel, S. Bajt, L. Redecke, H. N. Chapman, A. Meents, D. Turk, W. Hinrichs, T. J. Lane#
* these authors contributed equally to this work
# correspondence: thomas.lane@desy.de
Reference
- Y. A. Reinke & R. Schubert et al.
“SARS-CoV-2 Mpro responds to oxidation by forming disulfide and NOS/SONOS bonds”
Nature Communication, Volume 15, 3827 (2024).
doi:10.1038/s41467-024-48109-3
https://www.nature.com/articles/s41467-024-48109-3
