XFEL: Unlocking secrets of two vital proteins
Unlocking secrets of two vital proteins
Myoglobin 3D structure. Public domain image
Scientists have used the European XFEL instrument for Femtosecond X-ray Experiments (FXE) to reveal hidden secrets of two proteins that are vital for proper cellular functioning: nitrosyl-myoglobin and cytochrome c. An international team of scientists that carried out the study was led by researchers from École polytechnique fédérale de Lausanne (EPFL), Switzerland, in collaboration with scientists from Japan, UK, Poland and the European XFEL. The team has published two papers: the first in Nature Communications focusing on nitrosyl-myoglobin and the second in Proceedings of the National Academy of Sciences of USA focusing on cytochrome c.
Nitrosyl-myoglobin and cytochrome c, are both members of a group of proteins known as heme proteins. These proteins are responsible for key cellular processes, such as transporting and storage of small molecules in our blood and muscles, and also transmitting signals in our brains and facilitating enzymatic reactions. On an atomic level, myoglobin binds and releases oxygen, carbon dioxide or nitric oxide. Cytochrome c participates in electron transport inside cells, which is part of the pathway for synthesis of ATP, the energy-carrying molecule found in the cells of all living things. The description of how those proteins behave during these fundamental biochemical reactions is of extreme importance for understanding how the human body works.
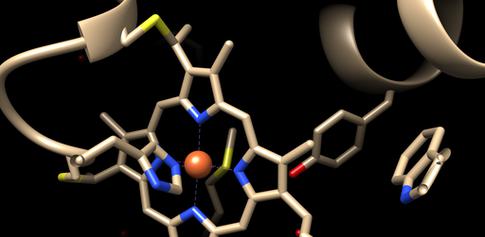
The structure of the myoglobin is known to change from a “relaxed” planar state, to a highly domed “tense” configuration when binding and releasing the small molecules and it has, until now, been unclear what exactly causes this transition and the intermediate steps between them. Moreover, it was not known if this structural change was a universal behaviour to all heme proteins, especially those involved in transportation, storage and electron transfer such as myoglobin, hemoglobin and cytochrome c.
It was the details of these processes that the scientists wanted to get to grips with. European XFEL scientist Frederico Lima, who was part of the team, has been involved in similar work since his doctoral studies. “I have a strong connection with this project as it was the main topic of my PhD thesis back in Switzerland. I feel very happy and honoured that 5 years after our first work on nitrosyl-myoglobin was published, I could witness the continuation of this work and help unravelling the details of ultrafast changes on myoglobin thanks to the capabilities provided by FXE.”
“The high number of X-ray flashes per second available at the European XFEL machine and femtosecond X-ray Emission Spectroscopy (XES) capabilities of the FXE instrument make it the perfect place to perform these kinds of investigations. We can now acquire superb data quality even from sensitive and dilute protein solutions, and this is very exciting,” says Dmitry Khakhulin, Acting Group Leader of the FXE team.
The researchers used the FXE instrument to artificially trigger the detachment of the small molecule or the electronic transfer by using the ultrashort optical laser pulses. They then used another short, hard X-ray pulse from the European XFEL to induce X-ray emission (XES), a very sensitive fingerprint of the spin state of molecules, which monitored the changes in the protein’s active centre (the heme). They could thus determine the change from relaxed planar to tense domed structure and back again is actually caused by a cascade among spin states.
Taken together the two studies show that the process of doming is not limited to myoglobin but also seen in other heme proteins such as cytochrome c. These studies are a step forward in understanding how cells work under healthy conditions, with possible future applications in health and medical sciences.
More Information:
A universal structural deformation in all heme proteins (News release from EPFL)
Unraveling the initial molecular events of respiration (News release from EPFL)
