XFEL: Using European XFEL to shed light on photosynthesis
Using European XFEL to shed light on photosynthesis
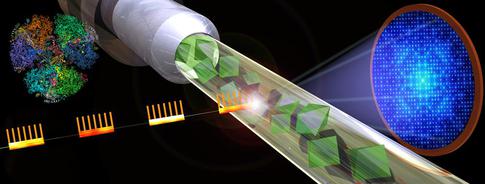
Graphic shows the basic design of a serial femtosecond crystallography experiment at European XFEL. X-ray bursts strike crystallized samples resulting in diffraction patterns that can be reassembled into detailed images. Copyright: Shireen Dooling for the Biodesign Institute at ASU
In a paper now published in Nature Communications an international group of scientists show that the fast X-ray pulse rate produced by the European XFEL can be used to study the structure of membrane proteins such as those involved in the process of photosynthesis. These results open up eagerly awaited experimental opportunities for scientists studying these types of proteins.
Large proteins and protein complexes are difficult to study with traditional structural biology approaches. Large protein complexes, such as those that sit across cell membranes and regulate traffic in and out of cells, are difficult to crystalize and generally only produce small crystals that are hard to analyse. The extremely fast X-ray pulses generated by European XFEL now enable scientists to collect large amounts of data from a stream of small crystals to develop detailed models of the 3D structure of these proteins.
Scientists have now, for the first time, been able to show how the fast X-ray pulse rate at European XFEL can be used to study the structure of large membrane proteins. The novel data presented in the study shows the structure of Photosystem I involved in photosynthesis in the so-called dark state, before being activated by light to start the process of converting light to energy. It is the first membrane protein to be successfully studied at the facility.
Prof. Petra Fromme from Arizona State University (ASU) in the USA who led the study said: “This work is so important, as it shows the first proof of concept of megahertz serial crystallography with one of the largest and most complex membrane proteins in photosynthesis: Photosystem I. The work paves the way towards time-resolved studies at the EuXFEL to determine molecular movies of the light-driven path of the electrons in photosynthesis or visualize how cancer drugs attack malfunctioning proteins.”
The scientists also describe how to produce large amounts of crystals of similar size and shape needed for this kind of experiments, and how crystal size influences data collection and analysis.
Prof. Adrian Mancuso, group leader at the SPB/SFX instrument where the experiments took place said: “Large proteins such as membrane proteins are difficult to study with traditional X-ray sources. We are pleased we have been able to demonstrate that the European XFEL can offer scientists an alternative way of shedding light onto these interesting and complex molecules.”
The study was a collaborative effort involving nearly 80 researchers from 15 institutions, including ASU, the European XFEL, DESY, the Center for Ultrafast X-ray Science, Hauptman-Woodward Institute, SUNY Buffalo, SLAC, University of Hamburg, University of Goettingen, Hungarian Academy of Sciences, University of Tennessee, Lawrence Livermore National Laboratory, University of Southampton, Hamburg University of Technology, University of Wisconsin.
-----
How it works:
For their experiments the scientists used the instrument for Single Particles, Clusters, and Biomolecules & Serial Femtosecond Crystallography (SPB/SFX) at European XFEL. The instrument is designed to use the extremely fast (femtosecond) intense X-ray pulses for imaging and crystallography experiments in order to explore the structure and dynamics of small biological samples.
For Serial Femtosecond Crystallography experiments a stream of small crystals is injected into the X-ray beam. The European XFEL X-ray laser is so powerful it will destroy the crystals, but not before information about the positions of the atoms in the protein have been recorded by the detector located behind the sample. Subsequent X-ray pulses will collect information from the crystals which are continually fed into the X-ray beam via a liquid jet.
-----
Original article:
Gisriel, C., Coe, J., Letrun, R. et al. Membrane protein megahertz crystallography at the European XFEL. Nat Commun 10, 5021 (2019) doi:10.1038/s41467-019-12955-3
-----
Read also the news release from the Biodesign Center for Applied Structural Discovery at the ASU.
