XFEL: Shining a light on antibiotic resistance
Shining a light on antibiotic resistance
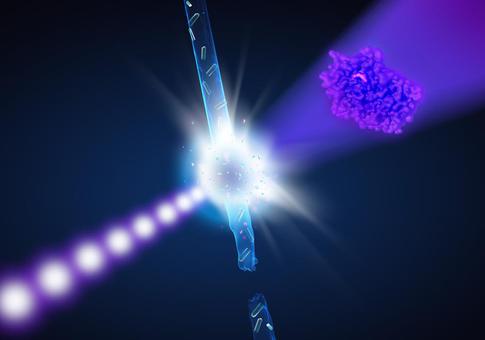
Artist's impression of the experiment: When the ultra-bright X-ray flashes (violet) hit the enzyme crystals in the water jet (blue), the recorded diffraction data allow to reconstruct the spatial structure of the enzyme (right). Credit: DESY/Lucid Berlin
A large international collaboration, including European XFEL scientists, has announced the results of the first scientific experiment run at European XFEL. A paper published today in the journal Nature Communications presents the results from the very first user experiment that took place in September 2017, just days after the facility was inaugurated. The results include details of the structure of an enzyme responsible for antibiotic resistance in the bacterium Klebsiella pneumoniae - a serious concern in hospitals worldwide. The paper is the second publication of research results obtained at the European XFEL.
“The groundbreaking work of the first team to use the European XFEL has paved the way for all users of the facility who greatly benefit from these pioneering experiments,” emphasises European XFEL managing director Robert Feidenhans'l. “We are very pleased - these results show that the facility works even better than we had expected and is ready to deliver new scientific breakthroughs.”
Principal investigator Dr. Anton Barty (left) from DESY and European XFEL scientist Dr. Richard Bean at the SPB/SFX instrument, setting up the first experiments at the new facility. Credit: DESY, Lars Berg
The group of over 120 researchers from over 30 institutions was led by DESY scientist Anton Barty from the Center for Free-Electron Laser Science (CFEL). The composition of the group and the experiment design was the result of open discussion involving scientists from across the scientific community and European XFEL staff. The aim was to allow everyone to participate, contribute, learn, and gain experience in how to carry out measurements. “Being at a totally new class of facility we had to master many challenges that nobody had tackled before,” said Barty. “I compare it to the maiden flight of a novel aircraft: All calculations and assembly completed, everything says it will work, but not until you try it do you know whether it actually flies.”
During this maiden flight, several aspects had to come together for the first time, including understanding how best to handle the increased number and frequency of X-ray pulses produced by the X-ray laser for the first time, designing and implementing novel sample delivery methods and the ensuring the custom made AGIPD detector could measure large amounts of data for the first time. Adrian Mancuso, leading scientist at the SPB/SFX instrument where the experiment took place said: “That everything came together so well in this first experiment is thanks to the hard work and drive of many people. These include the many experienced scientists involved, teams at DESY, the whole team at the SPB/SFX instrument and the many teams within European XFEL who support our work such as the detector and accelerator groups. Only by pooling everyone’s knowledge and expertise, and with everyone working hard together towards this common goal, could we make this work so well. I’m extremely pleased with how this turned out. It’s an excellent start to experiments at our new facility.”
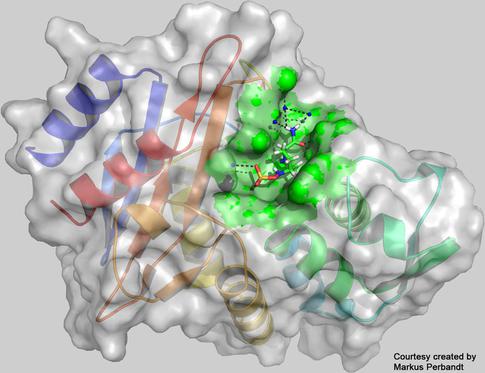
The three-diemnsional structure of the enzyme CTX-M-14 β-lactamase with the inhibitor avibactam bound to its active centre (green), as reconstructed from the measurements at the European XFEL. Credit: Universität Hamburg, Markus Perbandt
The researchers were able to collect enough data to reveal the structure of a hereto unknown protein structure: the enzyme known as CTX-M-14 β-lactamase from the bacterium Klebsiella pneumoniae whose multidrug-resistant strains are a concern in hospitals worldwide, together with an inhibitor known as avibactam. The enzyme works like a molecular pair of scissors cutting open penicillin derived antibiotics, thereby rendering them useless. To avoid this, antibiotics are often administered together with a compound called avibactam that blocks the molecular scissors of the enzyme. Unfortunately, bacteria with mutations that change the form of the scissors so that avibactam no longer prevents the bacteria enzyme from cutting open the antibiotics are now becoming more widespread. In their experiment the scientists used the X-ray laser to study the atomic structure of the non-mutated enzyme bound together with the avibactam compound. Both the avibactam and the enzyme structures were already known, however, the structure of the two molecules bound together is the first novel protein structure to be solved at the facility. In additional experiments the team plans to collect more data to create molecular movies showing the process of how the enzyme and avibactam molecule bind to each other. These details can inform the development of novel therapies.
DESY press release:
"First experiments at Europe's new X-ray laser reveal unknown structure of antibiotics killer"
Original article:
"Megahertz serial crystallography"; Max O. Wiedorn, Dominik Oberthür, Richard Bean, Robin Schubert, Nadine Werner et al.; "Nature Communications", 2018; DOI: 10.1038/s41467-018-06156-7
Read also:
"First European XFEL research results published"
